Standards

For an organization to prove that it meets the standard it has to undergo a management system audit, either internal or external. The question, therefore, is how can those utilizing compressed air effectively evaluate their assets’ performance as part of an ISO 50001 energy management system and, in doing so, grow their bottom line and minimize their negative environmental footprint.
[ Read Full Story ]
A Compressed Air & Gas Institute Q&A Session. Is a Variable Speed Drive (VSD) Compressor the Right Choice for Your Facility?
[ Read Full Story ]
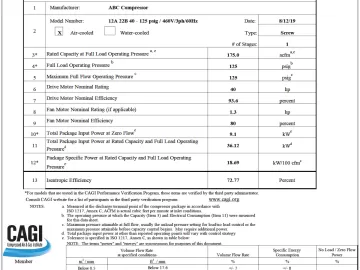
CAGI Performance Verification Program for Air Compressor and Dryer Selection
[ Read Full Story ]
Compressed Air Best Practices® Magazine interviewed Rick Stasyshan, Technical Consultant, of the Compressed Air & Gas Institute
[ Read Full Story ]
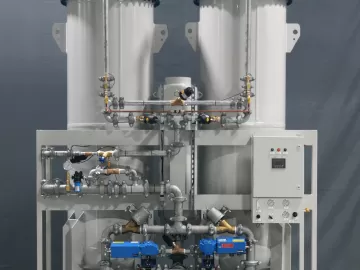
Transitioning to Oil-Free Compressed Air
[ Read Full Story ]
Hospital Air System Savings
[ Read Full Story ]
An Introduction to WAGD System Implementations
[ Read Full Story ]
BSA LifeStructures
[ Read Full Story ]
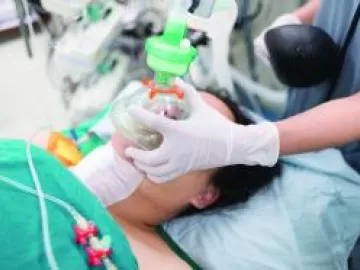
The Importance of Dewpoint for Medical Air Systems
[ Read Full Story ]
Supplied Air Respiratory Protection
[ Read Full Story ]