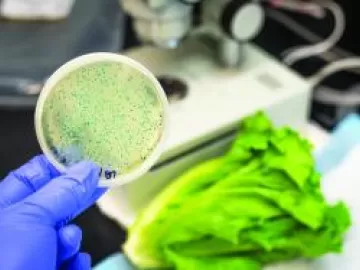
Sterile Compressed Air Filters Remove Bacteria
When compressed air comes into direct contact with a product, many applications believe their “standard” particulate and coalescing oil-removing filters, installed either side of a compressed air dryer, are sufficient to protect the downstream processes. Strangely enough, the removal of bacteria is often overlooked, despite this level of filtration being readily available, easy to procure, install and maintain.